VENNER
Lo Trach Cuff Pressure Controller
Rapid development for
life-saving control
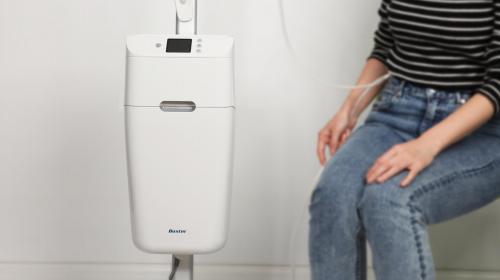
In under a year IDC managed the design, development and production of a ground-breaking Cuff Pressure Controller which works with LMA LoTrach™ Endotracheal and Tracheostomy tubes to prevent Ventilator Associated Pneumonia (VAP).
IDC designed an innovative and lifesaving Cuff Pressure Controller with phenomenal technological advances in engineering and design.
Venner had a series of issues that included inconsistent actuation of the pressure chamber, software bugs, and poor interface design.
IDC effectively managed to resolve these issues through careful root cause analysis, redesign and close coordination with Venner’s key stakeholders.
The work was managed across continents and to tight timescales with Venner’s project manager in Singapore, the manufacturing site in Germany and component suppliers from around the world.
By analysing the medical industry safety requirements, IDC delivered a manufacturing and testing solution resulting in a CE approved product currently selling in Europe.
Endotracheal and tracheostomy tubes include an inflatable cuff that is designed to seal against a patients’ trachea when they are ventilated and should prevent fluids entering into the lungs of patients, causing potentially fatal infections such as pneumonia.
Previously these cuff devices have only provided a partial seal that can allow secretions to pass down to the lungs.
The resulting design of the LMA LoTrach™ Cuff Pressure Controller is so effective that the LMA LoTrach™ tube system can be used for up to 30 days. With mains and battery power to the system, the CPC can also be used during patient transfer.
Trials have already proved how effective the product is and critical care patients should reap the benefits of the system with a reduction in lung infections and VAP.