ACTIVE NEEDLE
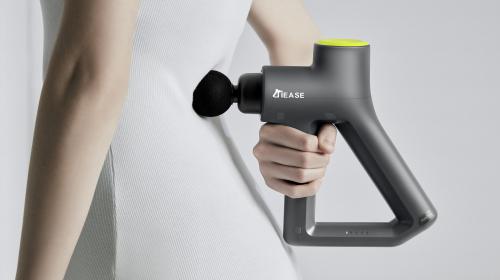
TranQuill® Tattoo Device
Reducing needle pain
with ultrasonics
TranQuill® offers a unique low-pain experience for patients through ultrasonic technology while providing a high quality experience for tattoo artists.
Harnessing the value of low-pain needle technology
Active Needle Technology, a company specialising in ultrasonic technology for needle-based medical procedures, initially focused on medical applications like ultrasound guided injections and biopsies. Their technology significantly reduced pain and skin trauma by using ultrasonic vibrations. However, they recognised the potential for broader applications, particularly in the tattoo market, where pain management could greatly enhance user experience. They chose to partner with IDC to develop the new product.
Weight, vibration & durability
Active Needle attempted to adapt their technology to create a tattoo device that would enable tattooing with reduced pain. However, they encountered two significant challenges:
Excessive weight and vibration: the ultrasonic transducer required to generate high-frequency vibrations was heavy and caused the device to vibrate excessively when combined with the reciprocating mass of the device. This meant the device vibrated so much that it could barely be held and could not be used to safely tattoo.
Wiring and durability issues: the transducer required power via wired connections, which broke quickly due to the rapid back-and-forth movement of the device at 9,000 RPM. The constant strain on the wires meant the device was unreliable and unusable.



System optimisation & user-centred design of handpiece and console
A motor capable of varying the power based on the mechanical load was added enabling the device to have a greater speed consistency to the millisecond. Custom-built parts, including a specially designed motor shaft with a welded hub, helped further refine the device to manage vibrations effectively.
Beyond mechanical challenges, we focused on creating a user-friendly design that would appeal to tattoo artists. Interviews were carried out with experienced artists to understand ergonomic needs and developed both the handpiece and a control console that fit within the artists’ work environment.
[We were delighted to have progressed this project with the valuable assistance of IDC. The project, part financed by InnovateUK, produced an advanced prototype that enabled the company to licence the technology to a leading tattoo company.
]


Extensive user testing with recorded pain score reduction
Extensive testing of the TranQuill® device demonstrated its effectiveness in reducing pain during tattooing. In trials, participants reported an average pain score reduction from 8 out of 10 (without ultrasound) to just 3 out of 10 (with ultrasound activated), showcasing a significant improvement in the tattooing experience. This project not only solved complex engineering challenges but also illustrated the potential of ultrasonics in new markets, with additional applications in medical tattooing for scars and burns under exploration. IDC’s journey highlights the importance of iterative design, user feedback, and innovative engineering solutions in creating ground-breaking products.

Our involvement in TranQuill®:
- Mechanical problem solving
- Vibration analysis
- Testing rig design and build
- Vibration testing and iteration
- Component specification
- Detailed design for manufacture
- Material specification
- Prototype manufacture
- Engineering drawings
- Industrial design & CMF
- Digi-physical user experience design
- Electronics hardware & software engineering
- Production support

Sign up to receive our top resources on product development and innovation.